Javier Terriente - 15 September 2022
How Artificial Intelligence Can Unlock Cures for Current and Future Diseases?
AI can address many challenges and limitations in traditional R&D and offer value in drug discovery in multiple ways: speeding up the process, reducing costs, and improving success rates in clinical phases.

Drug discovery scenario
We (bio)technological optimists believe that, with the right tools, we can achieve the ambitious goal of curing all diseases, stopping aging, and ultimately, bringing about improvements in quality of life to previously unimaginable levels. But being optimistic and being naïve are two completely different things. According to the Open Targets platform, which compiles comprehensive information derived from preclinical databases, scientific articles, and clinical studies, there are 23,000 diseases, with new ones being added annually, approximately 15,000 potential therapeutic targets, thousands of which have an unknown function in the context of hundreds of diseases, and 12,000 pharmacological molecules described for their interaction with targets, of which only 600 have reached the market.
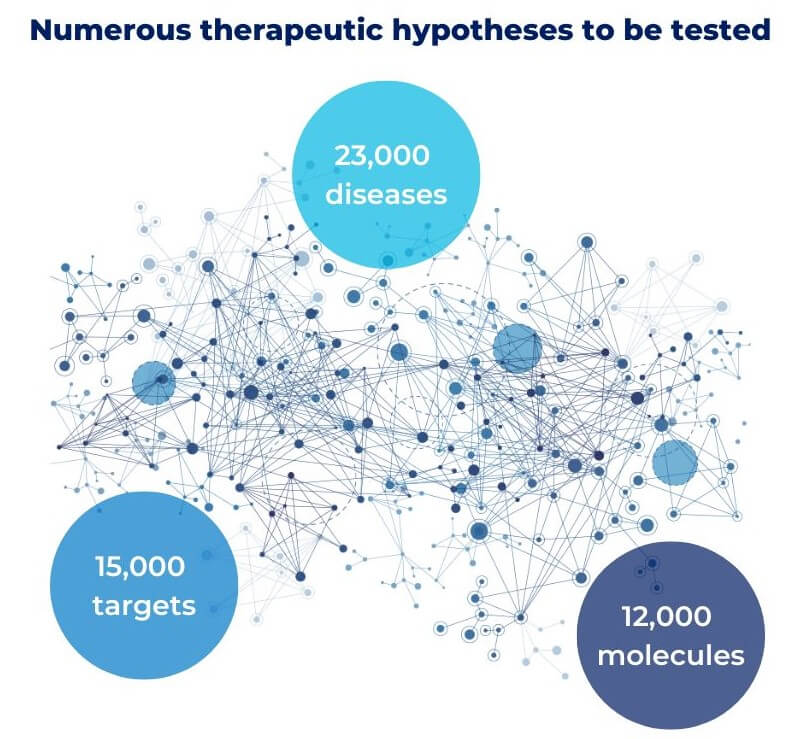
We can get the following conclusion from a straightforward mathematical exercise based on the combinatorial analysis of these numbers: there are countless therapeutic hypotheses to be tested. In other words, in a context in which a large number of rare and not-so-rare diseases do not yet have an available cure, there are millions of potential combinations between therapeutic targets and molecules that have not yet been explored.
However, discovering a new drug is not as simple as mixing two reagents in a liquid and checking to see if the color changes. It is a hard process, which requires meeting essential criteria:
- Find therapeutic targets whose pharmacological modulation can halt the disease's progression or reverse it.
- Design and develop powerful and selective molecules directed at these targets, to avoid producing toxicity due to off-target effects.
- Ensure that these compounds have suitable metabolic profiles and can access the tissue of interest in order to correctly execute their activity.
Meeting these criteria is difficult with current technology. It requires a thorough understanding of the biological processes affected by a disease, the identification and experimental validation of potential therapeutic targets against the disease under study, the screening of thousands of compounds (through in silico, in vitro, and in vivo assays) that can modulate the activity of these targets, and the selection of the most promising pharmacological candidates based on their toxicological and metabolic profiles as well as, of course, their preclinical (in animal models) and clinical (in humans) efficacy.
From the start of a project in the preclinical phase to its arrival in patients, this exhaustive procedure, to which thousands of biotech and pharmaceutical companies are dedicated, has a success rate of 4%, takes 12 years, and costs €1 billion. These metrics led the FDA to allow the introduction of 50 new medications to the market in 2021. The ambitious aim outlined above cannot be accomplished at the current pace in time for our generation and our children to benefit from it.
AI is changing drug discovery
That said, is there a shortcut that can improve success rates, speed up the process and reduce the huge costs involved in developing a new drug? Perhaps the solution lies in artificial intelligence (AI). Until a few years ago, only a few pioneering companies had embraced AI-based tools applied to drug discovery. Although it cannot be said the use of these tools have been democratized, the growth trend of new companies shows that the use of these tools is on its way to being essential. In fact, according to the latest study by Deep-pharma.tech 500 companies and 100 corporations declare using AI in their drug discovery processes during Q1 2022. Actually, the market for AI in drug discovery is predicted by the ResearchAndMarkets.com report to reach $4 billion by 2027, growing at a compound annual growth rate (CAGR) of 45.7% from 2022.

Its uses extend, although not exclusively, to the identification of new therapeutic targets and drugs that modulate them, optimizing the design and synthesis of these drugs, and predicting their toxicity or ADME profile. The first compounds discovered by the use of AI in some stages of the discovery process have entered the clinical stages in the past three years. Some examples are the molecules developed by Recursion for neurofibromatosis or by BenevolentAI for atopic dermatitis. But the advances in this field have become evident this year, with the entry into clinical phases of the first drug fully discovered by AI: from the identification of the target to the design of the drug that modulates it (effective, safe, and with a good metabolic profile). What is relevant about this drug, developed by Insilico Medicine to treat fibrotic processes, is that its preclinical development took only 30 months (including a clinical Phase 0) and cost $2.6 million, as opposed to the typical 5 years and 5 million (at a minimum) involving traditional preclinical development. In other words, the time and cost of preclinical development were reduced by half (or less).
ZeClinics bet on AI for R&D
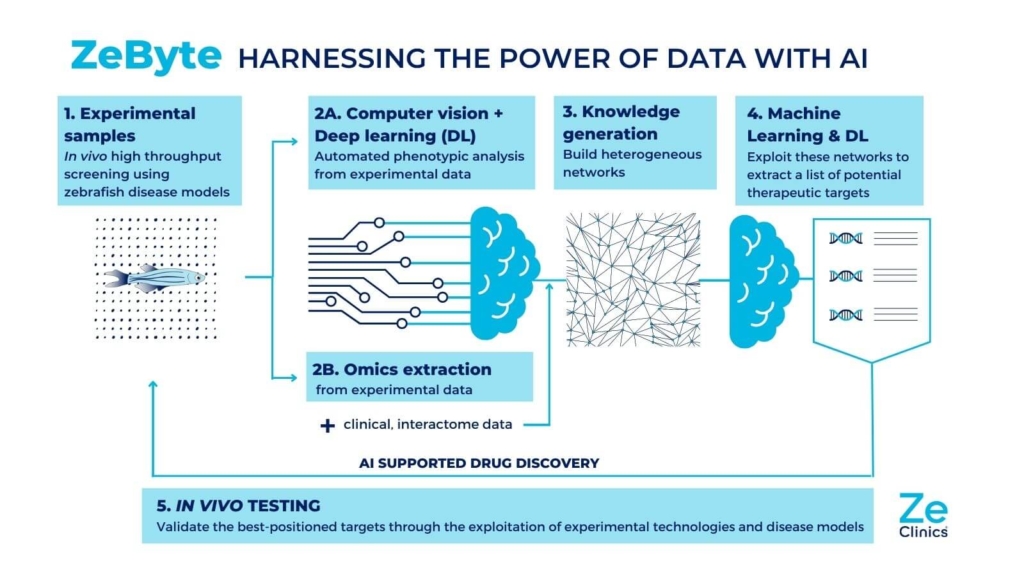
Although at less advanced stages of development, ZeClinics is also developing a drug and target discovery platform – ZeBYTE – that integrates AI tools into many of our zebrafish-based experimental processes. In this way, we use deep learning tools to automate the analysis of our experimental samples in order to extract relevant phenotypes; we use machine learning to build classifiers based on phenotypic data extracted from our zebrafish disease models or to predict drug toxicity; and we use deep learning and machine learning to interrogate the generated data matrices, which combine phenotypic and omics data, to identify new therapeutic targets and understand the potential mechanism of action of new molecules. We predict that this platform will enable us to find novel targets for the treatment of three different diseases in less than a year. And this is just the beginning.
Like ZeClinics, there are hundreds of companies around the world applying AI in their drug and target discovery processes. This suggests that more drugs bearing the IA seal will be approved for use in clinical trials in the upcoming years. Without a doubt, the preclinical phase already sees shorter deadlines and lower costs thanks to the usage of AI. The relevant question is whether these drugs will also display improved success rates during clinical phases. If they will be more effective, selective, and safe than those developed using conventional technologies. It is still early to predict if this will happen. But, for the common good, we hope that AI is the shortcut we were looking for to cure all diseases and even stop aging. As the matter stands now, it wouldn't be bad if someday we optimists were right.
REFERENCES
[1] Open Targets platform http://platform.opentargets.org/
[2] Artificial Intelligence for Drug Discovery. Landscape Overview Q1 202. Deep-pharma.tech https://www.deep-pharma.tech/ai-in-drug-discovery-2022-q1
[3] Artificial Intelligence (AI) in Drug Discovery Market by Component (Software, Service), Technology (ML, DL), Application (Neurodegenerative Diseases, Immuno-Oncology, CVD), End User (Pharmaceutical & Biotechnology, CRO), Region - Global forecast to 2024. ResearchAndMarkets.com https://www.researchandmarkets.com/reports/4858630/artificial-intelligence-ai-in-drug-discovery?utm_source=BW&utm_medium=PressRelease&utm_code=9kwcmv&utm_campaign=1719300+-+Worldwide+Artificial+Intelligence+(AI)+in+Drug+Discovery+Market+to+reach+%24+4.0+billion+by+2027+at+a+CAGR+of+45.7%25&utm_exec=como322prd
[4] Efficacy and Safety of REC-2282 in Patients With Progressive Neurofibromatosis Type 2 (NF2) Mutated Meningiomas (POPLAR-NF2). ClinicalTrials.gov identifier: NCT05130866. Updated June 15, 2022. Accessed August 04, 2022. https://clinicaltrials.gov/ct2/show/NCT05130866?term=REC-2282&cond=Neurofibromatosis+2&draw=2&rank=1
[5] A First-in-Human PoC Study With BEN2293 in Patients With Mild to Moderate Atopic Dermatitis. ClinicalTrials.gov identifier: NCT04737304. Updated April 08, 2022. Accessed August 04, 2022. https://clinicaltrials.gov/ct2/show/NCT04737304?term=BEN-2293&cond=Atopic+Dermatitis&draw=1&rank=1
[6] A Phase 1, Evaluate the Safety, Tolerability, and Pharmacokinetics of INS018_055 in Healthy Subjects. ClinicalTrials.gov identifier: NCT05154240. Updated March 24, 2022. Accessed August 04, 2022. https://clinicaltrials.gov/ct2/show/NCT05154240?term=Insilico+Medicine&cond=idiopathic+pulmonary+fibrosis&draw=2&rank=1
 By Javier Terriente
By Javier Terriente
Javier is a biochemist with a Ph.D. in developmental genetics. He has more than 20 years of research experience in the Academy and the biotech sector. In 2013, he co-founded ZeClinics. Recently, Javier co-founded ZeCardio Therapeutics. From 2013 to 2020 he was Chief Scientific Officer in ZeClinics, and now acts as Chief of Drug Discovery. Per his role in both companies, Javier manages a growing scientific team, has directed several Ph.D. theses and published multiple research articles, including several articles on the use of zebrafish for drug and target discovery. In addition to these roles, he serves as VP at Asebio, the Spanish association of biotech companies.