Vincenzo Di Donato - 24 November 2021
Zebrafish on the Move Towards Eye Disorders Research
Leveraging zebrafish to study Age-related Macular Degeneration

Previously in this blog, we have discussed the advantages of zebrafish for modeling obesity and other metabolic diseases. In the current article, we are going to explore the potential of zebrafish as a model for Age-related Macular Degeneration (AMD).
Eye Diseases: a present and future burden for the elderly population
Retinal diseases are among the main causes of severely impaired vision and blindness. They have steadily increased in Europe and the United States over the last few years, representing a serious socio-economic burden [1,2]. According to recent population-based epidemiological studies, AMD is the retinal disorder with the highest prevalence in older individuals [1]. AMD is characterized by progressive thinning of the eye macula, the part of the retina responsible for our central vision and thus enabling a clear vision and the ability to distinguish fine details. Eventually, patients affected by AMD suffer from vision loss which, in turn, leads to reduced independence and drastically compromises the quality of life [3-5]. The intense demographic changes, due to population aging, that western countries are facing suggest that by 2050 one out of four older adults in the EU will suffer from AMD [2]. Thereafter, the development of prevention and treatment strategies is crucial to avoid the expected pressure on national healthcare systems.
A zebrafish-based platform for testing therapeutics for retinal disorders
During the last decades, the Zebrafish (Danio rerio) has greatly contributed to the eye research field for several reasons. First, anatomical organization and circuit function of the retina are remarkably conserved between zebrafish and humans [6]. Second, the zebrafish retina, unlike that of mammals, displays a robust regenerative response after injury. Thus, it can be used to dissect the molecular mechanisms underlying functional and anatomical recovery of damaged tissue [7].
Death by apoptosis of photoreceptor cells, responsible for visual phototransduction, is the final step of several retinal dystrophies with different underlying physio-pathologic mechanisms, such as AMD and Retinitis pigmentosa. Experimental animal models of these pathologies have been developed and are mainly based on light-induced retinal degeneration [8-10]. Through this approach, constant light irradiation induces thinning of the retinal Outer Nuclear Layer (ONL), where rod and cone photoreceptors reside, and, consequently, loss of these cells. Nevertheless, the reported experimental procedures vary between research groups and result in a diverse degree of severity of the observed phenotypes. Indeed, the establishment of a standardized protocol is needed.
At ZeClinics, we have developed a robust experimental pipeline for AMD modeling, based on the light-induced degeneration of adult zebrafish. The procedure consists of constant light irradiation and posterior sectioning and analysis of the ONL of sectioned retinae. We have defined as key parameters analyzed to evaluate the status of photoreceptor cells, the thickness, and the number of cells in defined retinal areas. Indeed, our assay is able to induce strong retinal tissue damage and photoreceptor apoptosis resulting in a significant reduction of ONL thickness and cell number per area of retinae of animals exposed to light compared to non-exposed animals (Figure 1). Thus, our zebrafish model recapitulates the major outcomes of AMD pathophysiology.
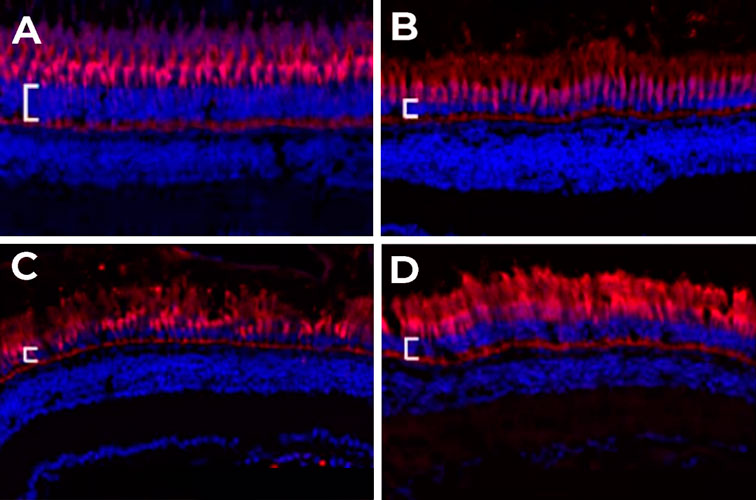 Figure 1. Retinal degeneration model: photoreceptors (red) and nuclei (blue) staining in retinal cryosections of adult zebrafish eyes. A) Negative control: fish not exposed to constant light (without retinal damage); B) Fish exposed to 60 h of constant light (Light-Induced Degeneration, LID); C) Fish exposed to 60h of constant light (LID) upon injection with vehicle Phosphate Buffer Saline (PBS); D) Fish exposed to 60 h LID upon injection with an active compound that protects from regeneration. The Outer Nuclear Layer (ONL) is marked with a white bar.
Figure 1. Retinal degeneration model: photoreceptors (red) and nuclei (blue) staining in retinal cryosections of adult zebrafish eyes. A) Negative control: fish not exposed to constant light (without retinal damage); B) Fish exposed to 60 h of constant light (Light-Induced Degeneration, LID); C) Fish exposed to 60h of constant light (LID) upon injection with vehicle Phosphate Buffer Saline (PBS); D) Fish exposed to 60 h LID upon injection with an active compound that protects from regeneration. The Outer Nuclear Layer (ONL) is marked with a white bar.
The use of therapeutic compounds administered via eye injection has been increasing over the last two decades as a medical practice for the treatment of retinopathies [11]. Rabbits and rodents are the most commonly used animals for intravitreal (IV) drug delivery and pharmacokinetics [12,13], while the use of zebrafish to test the administration of therapeutic or neuroprotective compounds is scarce [9]. Therefore, we have validated a protocol for IV injection of compounds of interest in zebrafish adult eyes. This procedure allows screening molecules with potential neuroprotective effects in an AMD model (as shown in Figure 2) and other ocular diseases.
 Figure 2. ONL cell thickness and cell number evaluation on four different conditions. Negative control (fish not exposed to constant light, without retinal damage); Fish exposed to 60 h LID; Fish exposed to 60 h LID upon injection with vehicle Phosphate Buffer Saline (PBS); Fish exposed to 60 h LID upon injection with an active compound that protects from regeneration. *** p<0.001.
Figure 2. ONL cell thickness and cell number evaluation on four different conditions. Negative control (fish not exposed to constant light, without retinal damage); Fish exposed to 60 h LID; Fish exposed to 60 h LID upon injection with vehicle Phosphate Buffer Saline (PBS); Fish exposed to 60 h LID upon injection with an active compound that protects from regeneration. *** p<0.001.
The combination of validated disease models and adequate drug administration procedures makes zebrafish a powerful alternative model for testing the efficacy of novel potential IV-injection-based treatment of retinal diseases, with the possibility of medium-throughput assessment of toxicity and pharmacokinetics.
Finally, our expertise in the analysis of the retinal tissue allows combining our ophthalmology platform with our genetics platform, enabling the generation of zebrafish overexpressing a mutant form of a gene of interest in a retinal-specific fashion or carrying a loss of function mutation in an eye-disease associated gene followed by retinal specific phenotypic analysis or transcriptomics. These approaches would help you to gain insights into molecular mechanisms underlying pathogenesis and unravel potential therapeutic strategies.
REFERENCES
[1] Rosenblatt TR, Vail D, Saroj N, Boucher N, Moshfeghi DM, Moshfeghi AA. Increasing Incidence and Prevalence of Common Retinal Diseases in Retina Practices Across the United States. Ophthalmic Surg Lasers Imaging Retina. 2021 Jan 1;52(1):29-36. https://doi.org/10.3928/23258160-20201223-06
[2] Li JQ, Welchowski T, Schmid M, Mauschitz MM, Holz FG, Finger RP. Prevalence and incidence of age-related macular degeneration in Europe: a systematic review and meta-analysis. Br J Ophthalmol. 2020 Aug;104(8):1077-1084. http://dx.doi.org/10.1136/bjophthalmol-2019-314422
[3] Nyman SR, Gosney MA, Victor CR. Emotional well-being in people with sight loss: Lessons from the grey literature. British Journal of Visual Impairment. 2010;28(3):175-203. https://doi.org/10.1177/0264619610374171
[4] van der Aa HP, van Rens GH, Comijs HC, Bosmans JE, Margrain TH, van Nispen RM. Stepped-care to prevent depression and anxiety in visually impaired older adults--design of a randomised controlled trial. BMC Psychiatry. 2013 Aug 9;13:209. https://doi.org/10.1186/1471-244X-13-209
[5] Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006 Dec 21;4:97. https://doi.org/10.1186/1477-7525-4-97
[6] Richardson R, Tracey-White D, Webster A, Moosajee M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye (Lond). 2017 Jan;31(1):68-86. https://doi.org/10.1038/eye.2016.198
[7] Wan J, Goldman D. Retina regeneration in zebrafish. Curr Opin Genet Dev. 2016 Oct;40:41-47. https://doi.org/10.1016/j.gde.2016.05.009
[8] Donovan M, Carmody RJ, Cotter TG. Light-induced photoreceptor apoptosis in vivo requires neuronal nitric-oxide synthase and guanylate cyclase activity and is caspase-3-independent. J Biol Chem. 2001 Jun 22;276(25):23000-8. https://doi.org/10.1074/jbc.M005359200
[9] Saito Y, Tsuruma K, Shimazawa M, Nishimura Y, Tanaka T, Hara H. Establishment of a drug evaluation model against light-induced retinal degeneration using adult pigmented zebrafish. J Pharmacol Sci. 2016 Jul;131(3):215-8. https://doi.org/10.1016/j.jphs.2016.05.009
[10] Sudharsan R, Simone KM, Anderson NP, Aguirre GD, Beltran WA. Acute and Protracted Cell Death in Light-Induced Retinal Degeneration in the Canine Model of Rhodopsin Autosomal Dominant Retinitis Pigmentosa. Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):270-281. https://doi.org/10.1167/iovs.16-20749
[11] de Vries VA, Bassil FL, Ramdas WD. The effects of intravitreal injections on intraocular pressure and retinal nerve fiber layer: a systematic review and meta-analysis. Sci Rep. 2020 Aug 6;10(1):13248. https://doi.org/10.1038/s41598-020-70269-7
[12] Del Amo EM, Urtti A. Rabbit as an animal model for intravitreal pharmacokinetics: Clinical predictability and quality of the published data. Exp Eye Res. 2015 Aug;137:111-24. https://doi.org/10.1016/j.exer.2015.05.003
[13] Schmitt M, Hippeläinen E, Raviña M, Arango-Gonzalez B, Antopolsky M, Vellonen KS, Airaksinen AJ, Urtti A. Intravitreal Pharmacokinetics in Mice: SPECT/CT Imaging and Scaling to Rabbits and Humans. Mol Pharm. 2019 Oct 7;16(10):4399-4404. https://doi.org/10.1021/acs.molpharmaceut.9b00679
 By Vincenzo Di Donato
By Vincenzo Di Donato
Vincenzo Di Donato is an Italian researcher, expert in genetics and molecular biology. After a master’s degree in molecular biology at the University of Naples, he obtained his PhD in neuroscience from the Curie Institute in Paris, where he chose the zebrafish as an animal model to study the development of the visual system of vertebrates. Through his work, he has helped establish innovative CRISPR/Cas9-based gene editing techniques. In 2017 Vincenzo joined the ZeClinics team in Barcelona as a postdoc to subsequently fill the position of head of the genetics area as of 2019. Since November 2020, he has held the position of scientific director of the company.