 Developmental Teratogeny
Developmental Teratogeny
Developmental Toxicity assay is an optimized method for an enhanced prediction and description of teratogenicity. The broad number of phenotypes tested and the accurate quantification of subtle morphometric changes provide an integral teratogenic profile of your chemical candidate.
Advantages
Predictability levels referred to rodents and humans above 80%.
Automated platform to process high amounts of larvae.
Mathematical-based approach that increases objectivity of verdicts.
Evaluation of an extensive panel of phenotypes.
Estimation of teratogenic potencies through teratogenic indexes.
Good Laboratory Practice (GLP) like studies.
Method description
Incubation of embryos with 5 different concentrations of the molecule of interest spaced by a constant factor 2 from 0 to 96 hours post fertilization (hpf). Subsequent automated imaging and evaluation of teratogenicity through a validated panel of phenotypes. Finally, the relationship between mortality and teratogenicity is calculated to determine teratogenic potency (teratogenic index).
Readouts
Determination of the following parameters at 24 and 96 hpf:
- LOEC: Lowest Observable Effect Concentration
- LC50: Lethal Concentration 50
Determination of the following parameters at 96 hpf:
- EC50: Effective Concentration 50 for each phenotype
- TI: Teratogenic index
 Figure 1. Concentration-effect curves for teratogenicity (light blue) and mortality (dark blue) at 96 hpf after exposure of embryos to a toxic compound.
Figure 1. Concentration-effect curves for teratogenicity (light blue) and mortality (dark blue) at 96 hpf after exposure of embryos to a toxic compound.
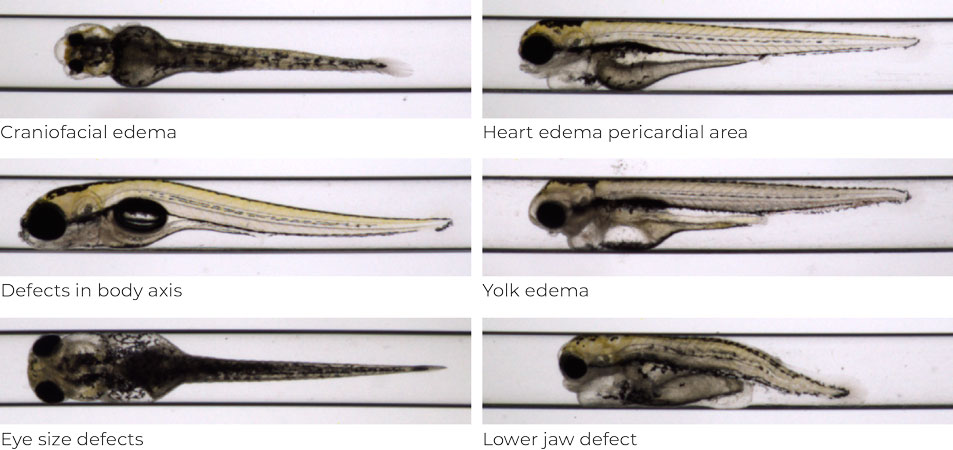 Figure 2. Phenotypical panel: examples of developmental defects in embryos exposed to different toxic compounds.
Figure 2. Phenotypical panel: examples of developmental defects in embryos exposed to different toxic compounds.
We'd like to hear from you
If you want more information about our DevTox
assay or have any other questions, please
contact our experts.
References
- Jarque S, Rubio-Brotons M, Ibarra J, Ordoñez V, Dyballa S, Miñana R, Terriente J. 2020. Morphometric analysis of developing zebrafish embryos allows predicting teratogenicity modes of action in higher vertebrates. Reprod Toxicol. 96:337–348.