 Zebrafish Crispants Service: Rapid Gene Knockout
Zebrafish Crispants Service: Rapid Gene Knockout
ZeClinics' zebrafish Crispants are a fast-track choice for screening loss-of-function alleles, and validating functional genetic models before committing to building a stable line.
The Crispant approach provides early proof-of-concept or viability testing of your gene knockout model in as little as one month.
Our zebrafish Crispants will enable you to reduce costs, time and animal use in your gene knockout projects.
Why Choose ZeClinics's Zebrafish Crispants Service?
Employing conventional CRISPR methodologies to generate a stable knockout model requires months of propagation and screening to stabilize and confirm the edit before assaying.
This investment of time and resources is incompatible with the fast pace of many clients' projects.
In order to address the long timelines associated with traditional genetic models, ZeClinics offers the Crispant approach.
Make critical decisions about your genetic or disease modeling strategy in a fraction of the time it takes to create an animal model.
Our Crispants Approach
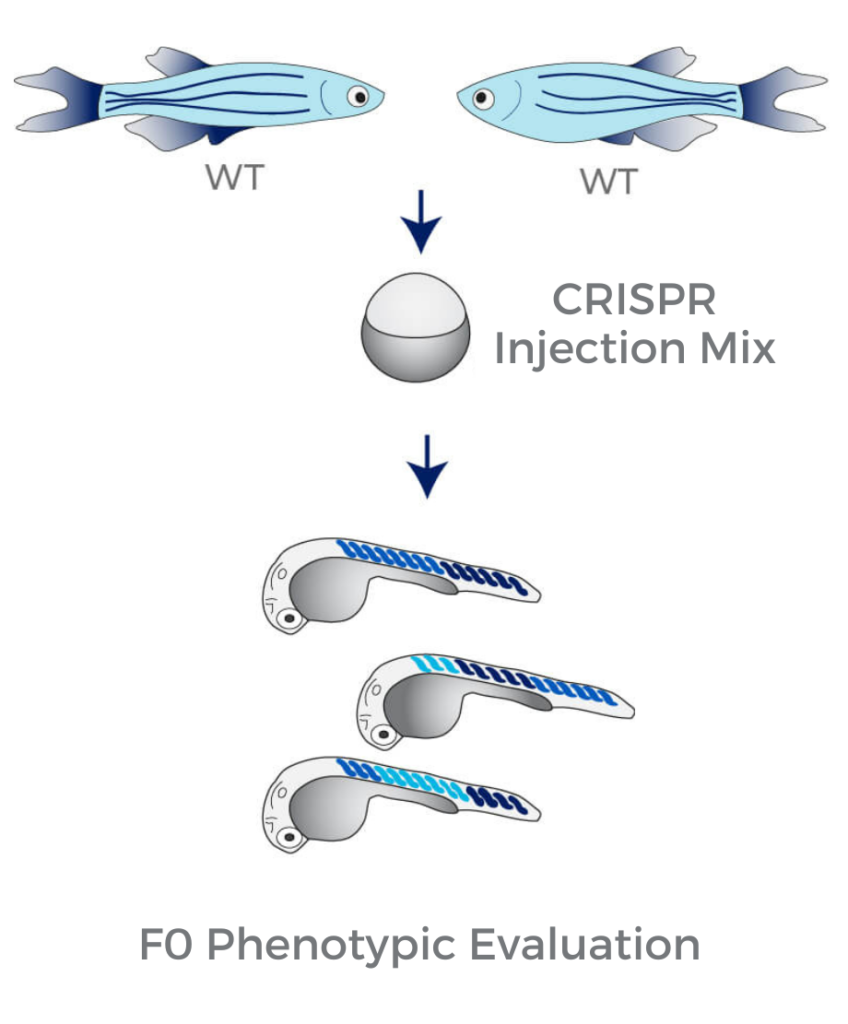
We efficiently "disrupt" the gene of interest using sgRNAs and the CRISPR technology. Within less than one month, we generate highly penetrant F0 knockouts carrying a mosaic set of somatic and germline mutations.
Due to the high mutagenesis rate in Crispants (close to 100%), the resulting loss-of-function phenotypes can be assayed immediately in F0 larvae.
Using the Crispant approach, it takes as little as a couple of weeks to go from gene disruption to behavioral phenotype instead of more than six months using standard CRISPR gene editing approaches.
Deliverables
Because the Crispant methodology creates mosaic F0 somatic knockouts, phenotypic analysis is performed by ZeClinics and zebrafish larvae are not delivered to the client. Based on the selected phenotypic screen, deliverables may include:
- Raw phenotypic data, including images.
- Expert data analysis and interpretation.
- Successful crispants can be recovered and converted to a knockout line build project.
Key Benefits of Crispants
Save time on "go/no-go" decisions: determine within a month whether a viable genetic model can be developed.
Scalable for high throughput screening and validation of candidate target genes. Evaluate more genetic targets in vivo in significantly less time.
Get a head start on full model creation while gathering early data: zebrafish crispants generated with this approach can be propagated to create a stable knockout line.
Edits are Permanent, unlike morpholinos, so animals can be assayed at all growth stages.
Applications of Zebrafish Crispants:
- Decide Early whether to invest time and resources into a viable genetic model.
- Quickly Screen Multiple Target Genes for biological function.
- Rapid Target Validation: determine whether your model has a disease specific phenotype.
- Easily Probe Functional Domains of your gene of interest.
- Gene Lethality Assessment
- Fast Disease Modeling
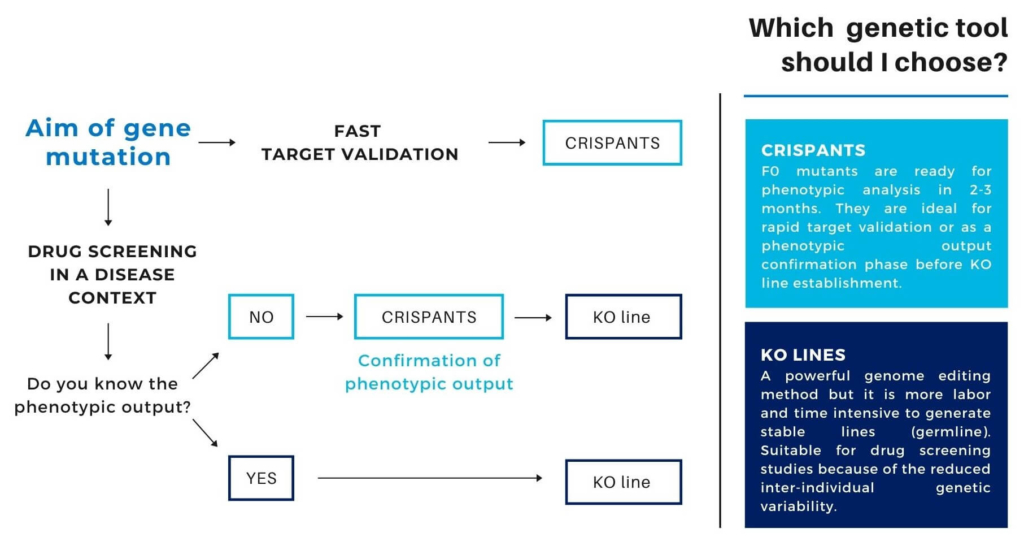
Feasibility of a Knockout Model
Crispants are interesting to confirm the expected phenotypic output of your custom mutation before establishing a zebrafish knockout line for further studies, such as drug screening.
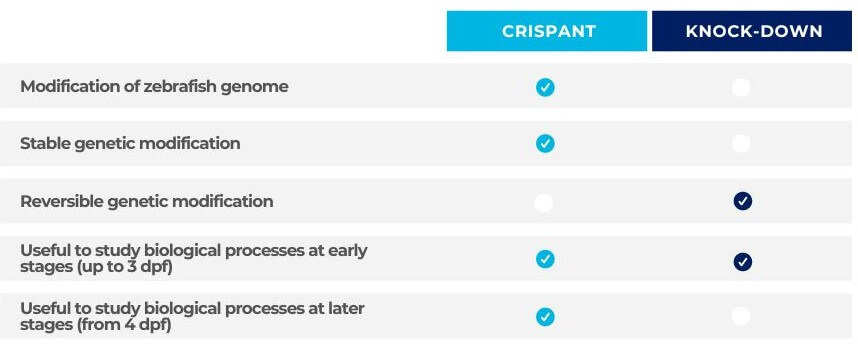
Comparison of Crispants vs Knockdown
Unlike transient knockdowns, such as morpholinos or ASOs, the genetic modifications of Crispants are permanent, so mutants can be assayed at all growth stages.
In addition, due to the stable gene editing, Crispants can be propagated to establish a stable knockout line.
We'd like to hear from you
If you want more information about our Crispants
generation services or have any other questions,
please contact our experts.
References
- Burger A, Lindsay H, Felker A, Hess C, Anders C, Chiavacci E, Zaugg J, Weber LM, Catena R, Jinek M, Robinson MD, Mosimann C. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development. 2016 Jun 1;143(11):2025-37.